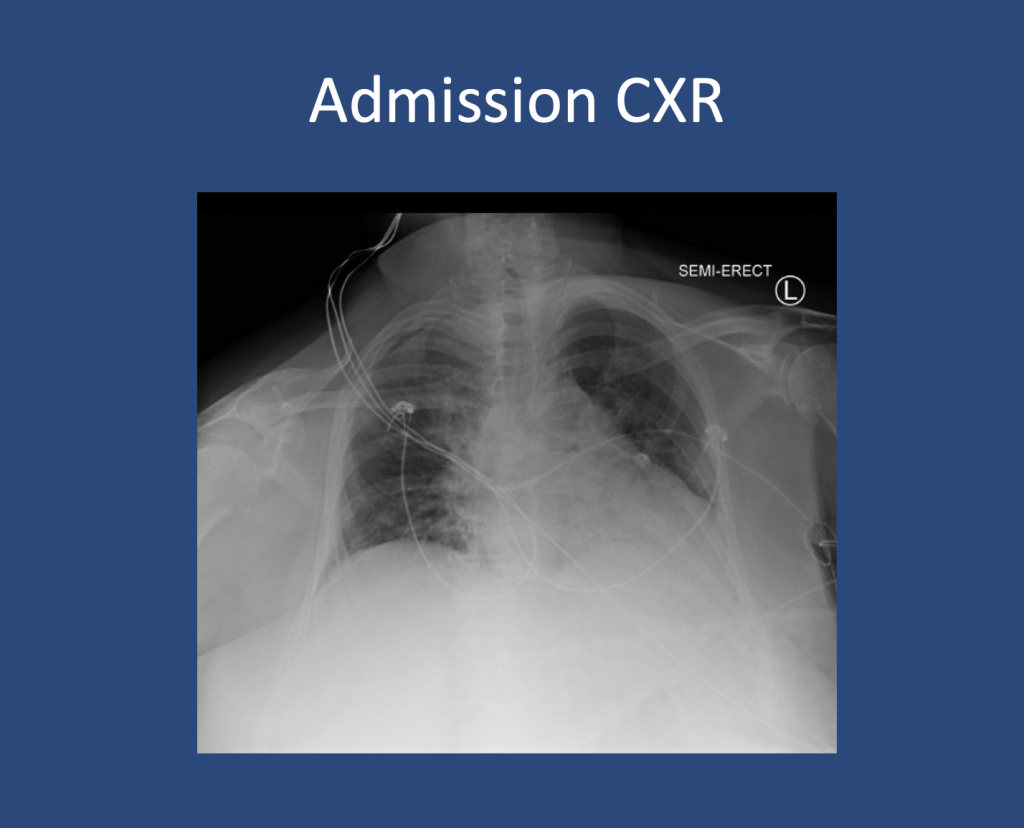
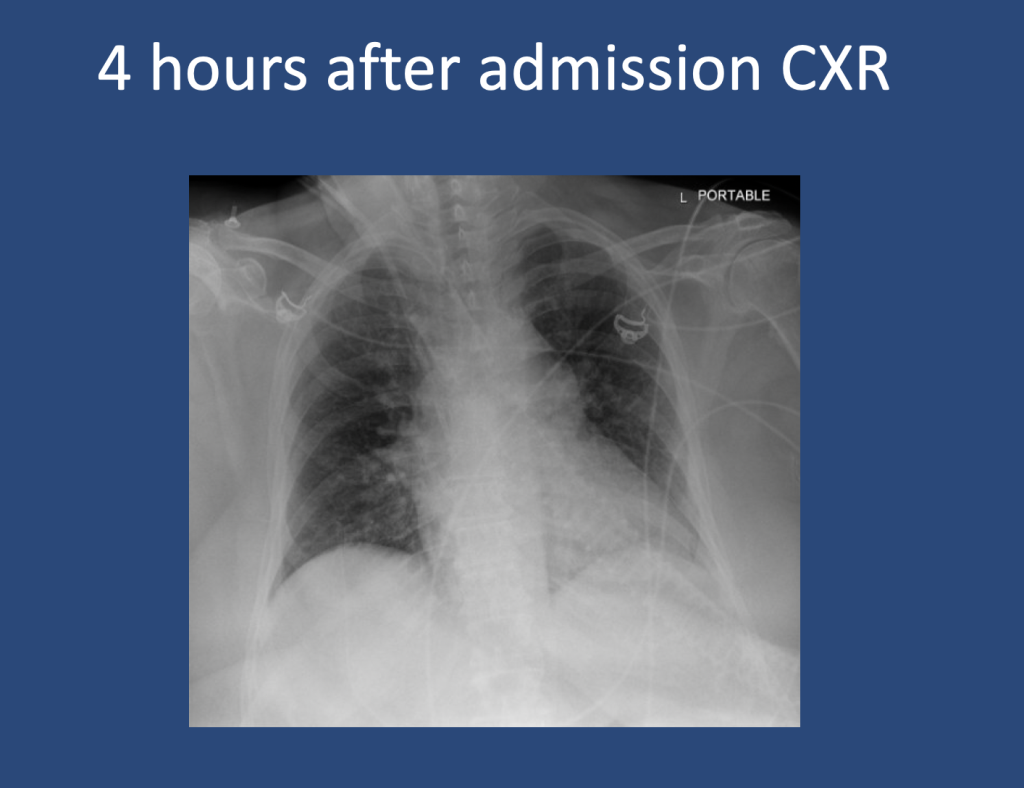
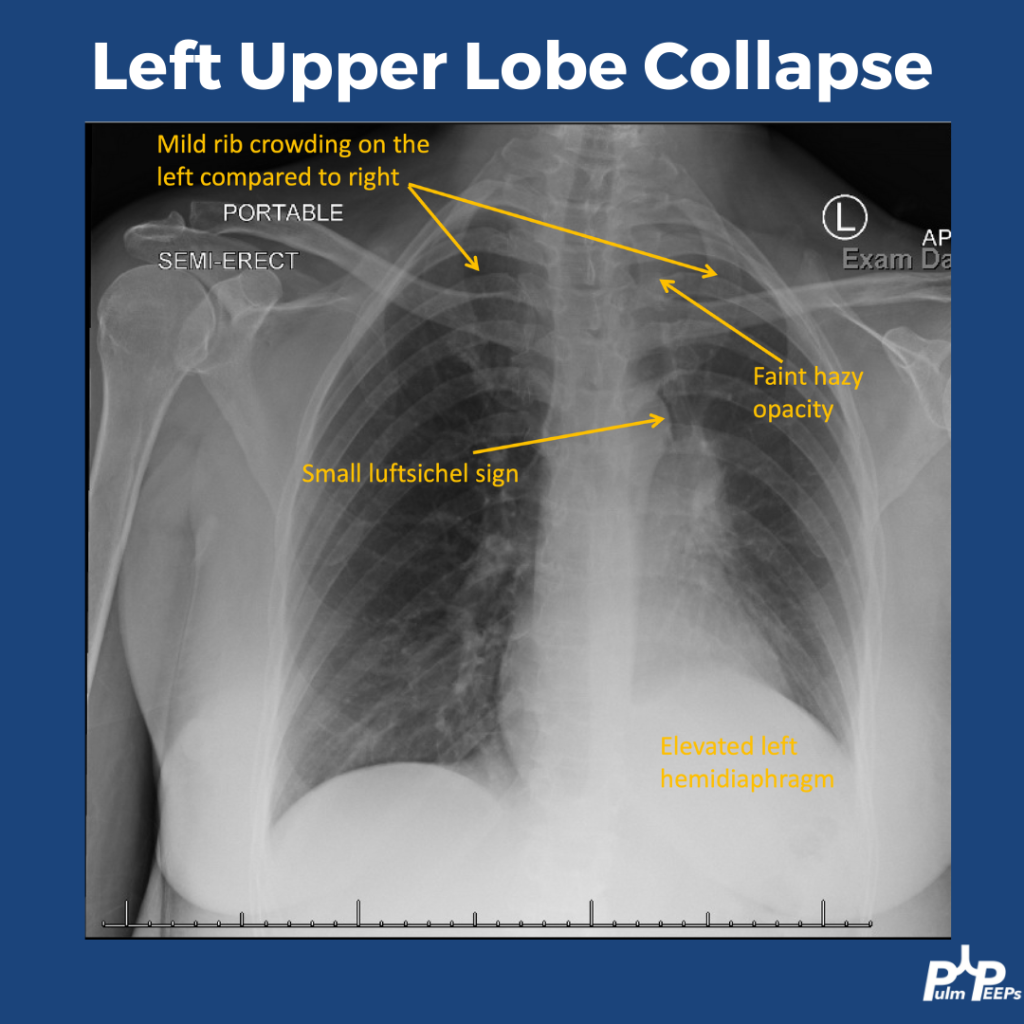
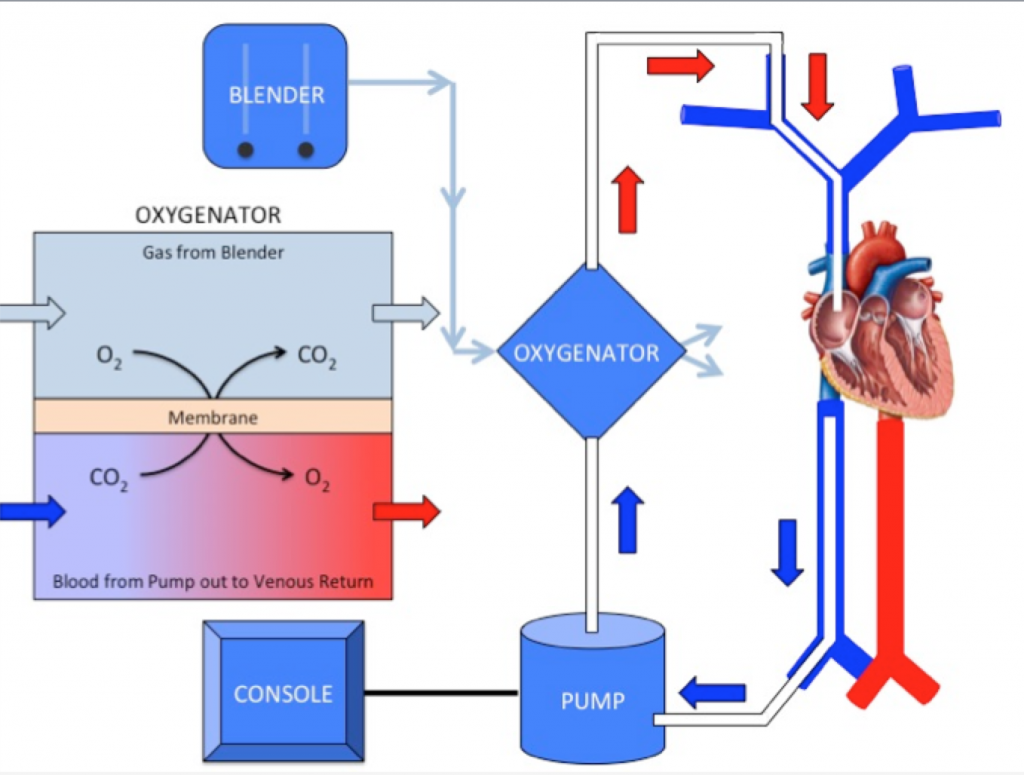
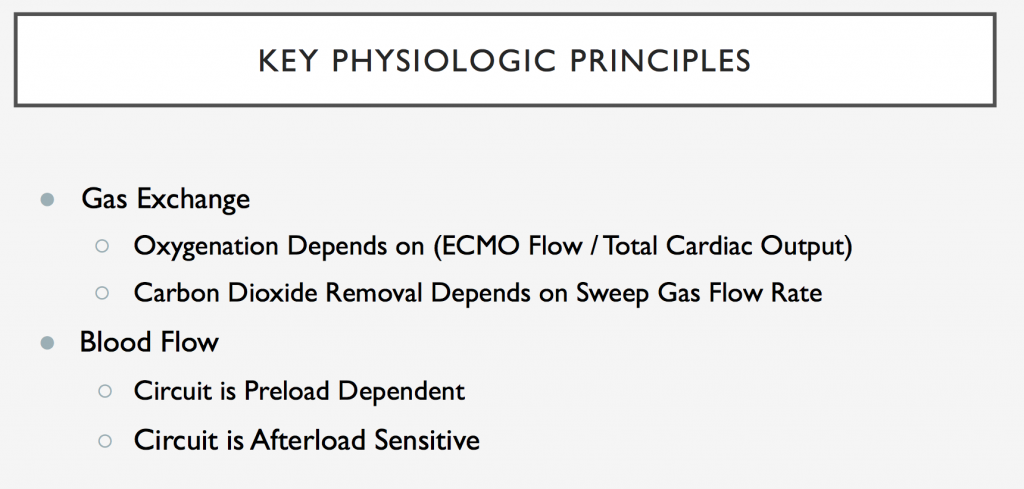
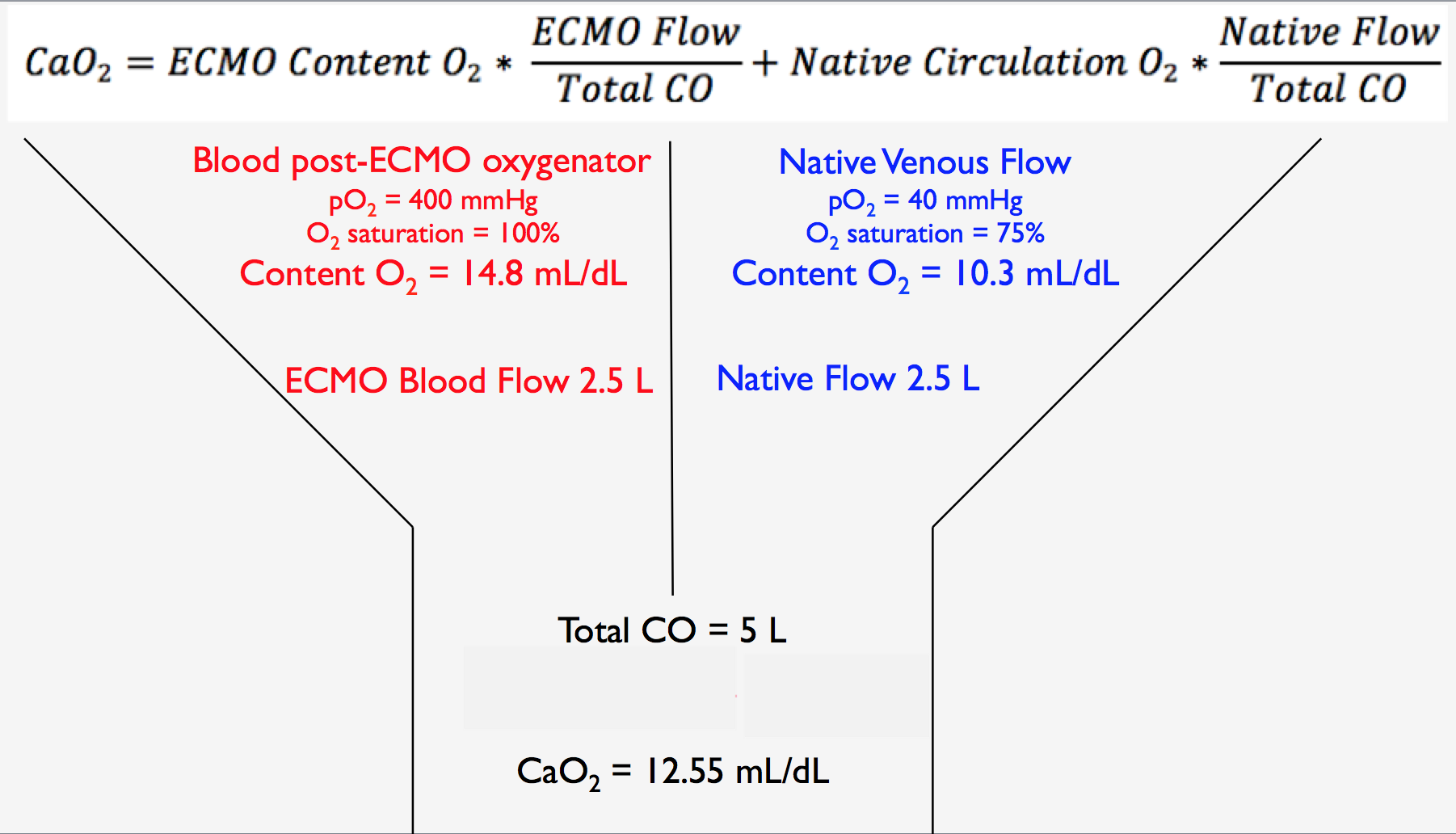
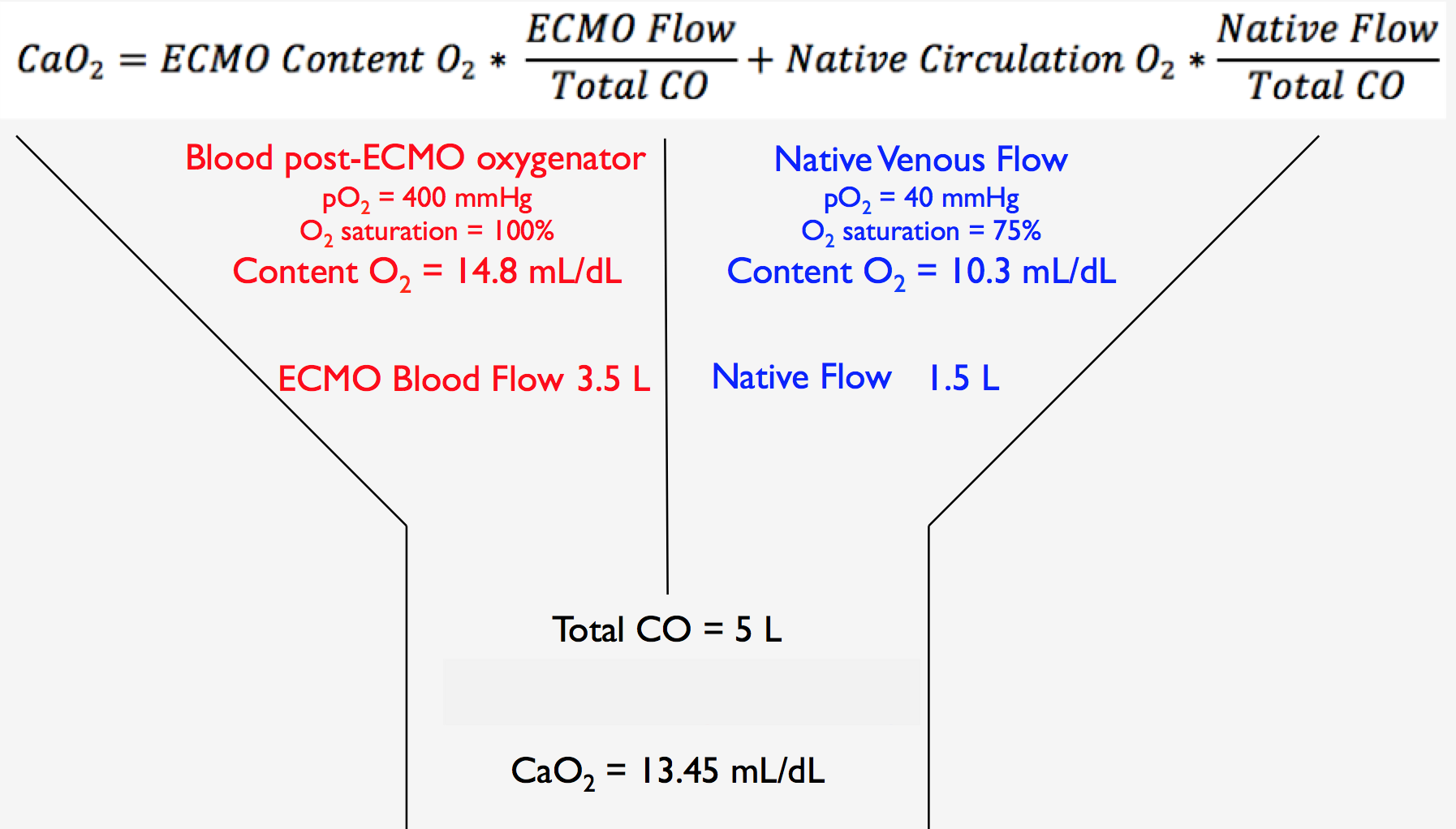
We are so excited to be launching a new series here at Pulm PEEPs! We’ll be talking about high yield topics in 15 minutes or less. In this series, Furf and Monty will tackle core points and provide an overview, key points, and further reading. We’re starting with a key point review of Immune Checkpoint Inhibitor Pneumonitis. Let us know if there are other topics you want to hear about!
Key Learning Points
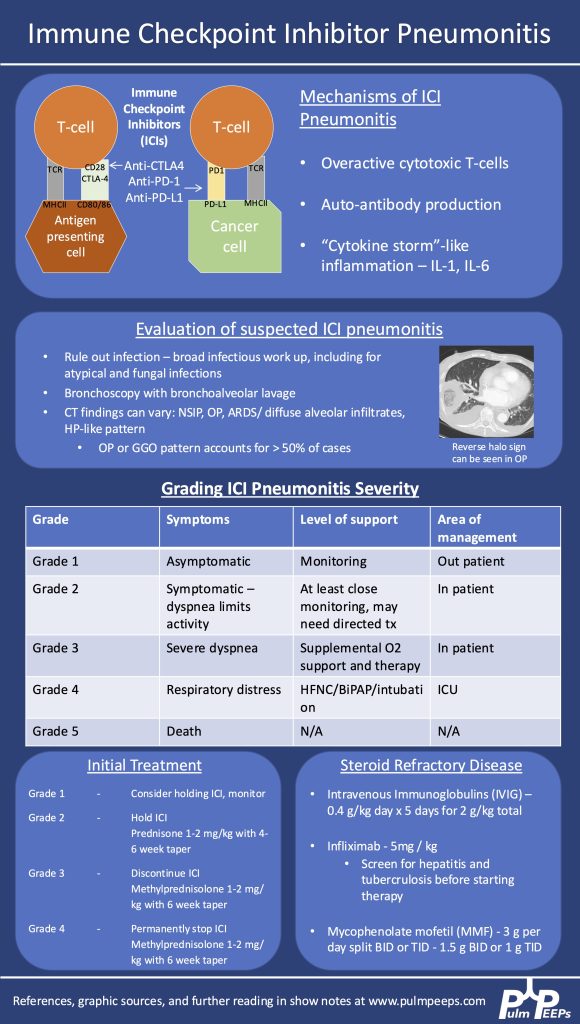
- Epidemiology & Pathophysiology
-
- Increasingly common as immunotherapy use grows in oncology.
- Caused by immune activation from PD-1, PD-L1, or CTLA-4 inhibitors.
- Mechanisms:
- Overactive T cells
- Autoantibody production
- Cytokine-mediated inflammation (e.g., ↑IL-1, ↑IL-6)
- Clinical Suspicion & Diagnosis
-
- Any new respiratory symptoms in a patient currently or previously on ICI → consider ICI pneumonitis.
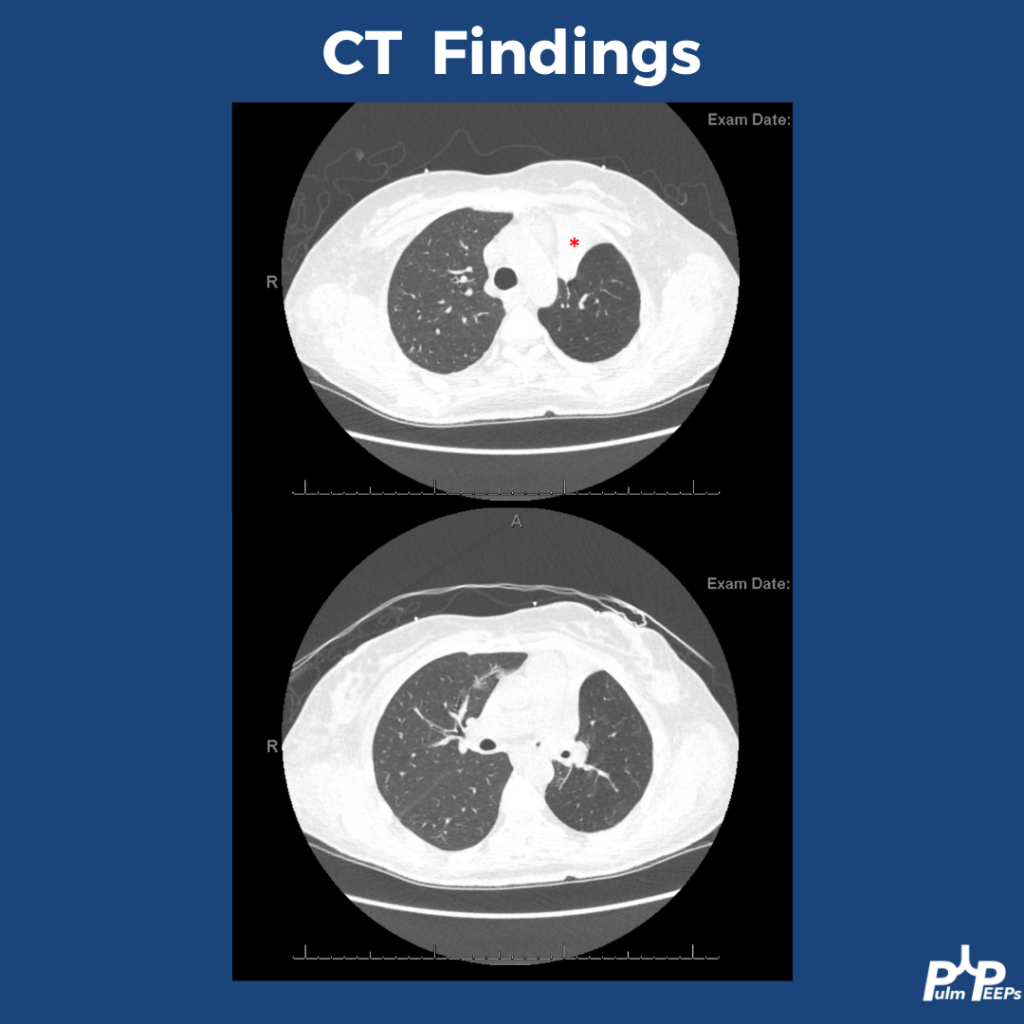
- CT findings are variable: can mimic organizing pneumonia, NSIP, ARDS, or diffuse ground glass opacities. Imaging pattern does not determine severity grade.
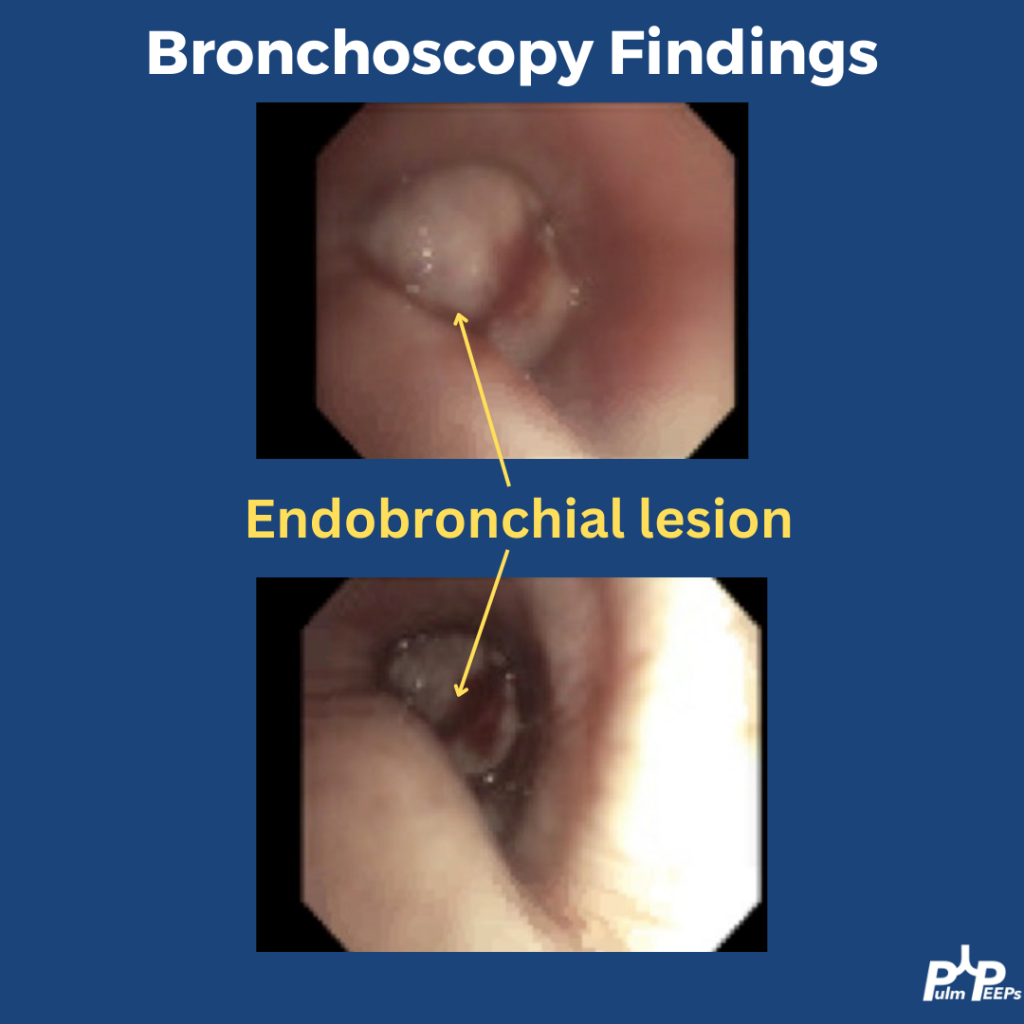
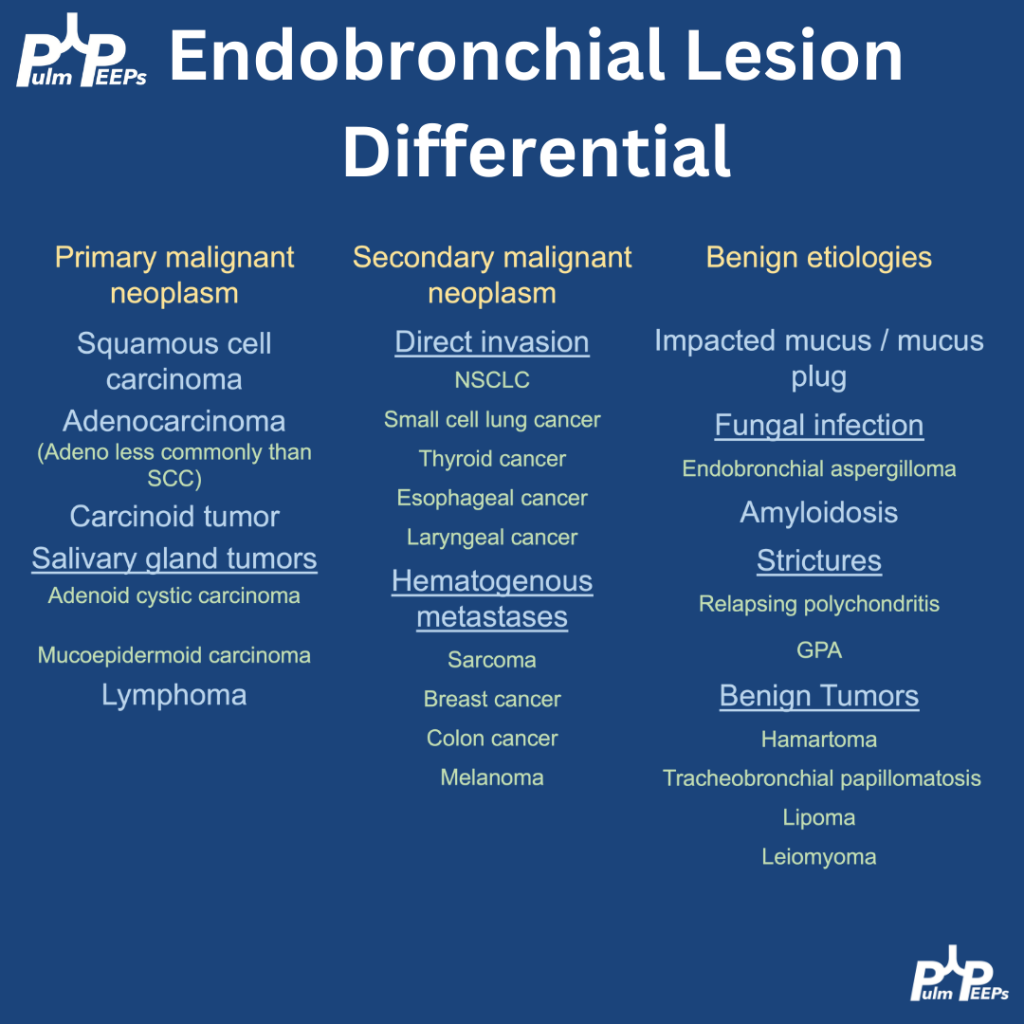
- Diagnosis is of exclusion — infection and malignancy progression must be ruled out first.
- Workup:
- Broad infectious evaluation (cultures, viral panel, fungal markers).
- Early bronchoscopy with BAL if feasible — typically lymphocyte-predominant in ICI pneumonitis.
- Screen for TB and hepatitis early (in case infliximab is needed).
- Severity Grading (Symptom- & O₂-based, not imaging-based)
-
- Grade 1: Asymptomatic → monitor, may hold ICI.
- Grade 2: Symptomatic but not hypoxic → prednisone 1 mg/kg/day PO.
- Grade 3–4: Hypoxemia or ICU-level care → methylprednisolone 1–2 mg/kg/day IV. Usually hold or permanently stop ICI.
- Steroid Management
-
- Typical taper: over 6 weeks for grade ≥3.
- Week 1: 1–2 mg/kg/day
- Gradual step-down to 0.25 mg/kg/day by week 5, then stop week 6.
- Chronic/recurrent cases may need slower tapers over months.
- Add GI prophylaxis and PJP prophylaxis during prolonged steroid use.
- Typical taper: over 6 weeks for grade ≥3.
- If Steroids Fail (no improvement after 48–72 hrs)
-
- Consider adding:
- IVIG (2 g/kg over 5 days)
- Infliximab (TNF-α inhibitor — requires TB/hepatitis screening)
- Mycophenolate mofetil (1–1.5 g/day BID or TID, start at effective dose quickly)
- IVIG may have lower mortality in some series but comes with risks (volume overload, thrombosis, infusion reactions).
- Consider adding:
- Emerging Therapies
-
- JAK inhibitors are under investigation as possible future options.
- Multidisciplinary Care
-
- ICU management is a team sport — coordinate with oncology, critical care, infectious disease, and pharmacy.
Infographic
References and Further Reading
- Managing Immune Checkpoint Inhibitor Pneumonitis in the ICU. Montemayor, Kristina et al.CHEST Critical Care, Volume 3, Issue 1, 100126
- Lavalle S, Masiello E, Valerio MR, Aliprandi A, Scandurra G, Gebbia V, Sambataro D. Immune checkpoint inhibitor therapy‑related pneumonitis: How, when and why to diagnose and manage (Review). Exp Ther Med. 2024 Jul 30;28(4):381. doi: 10.3892/etm.2024.12670. PMID: 39113908; PMCID: PMC11304171.
- Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev. 2019 Nov 6;28(154):190012. doi: 10.1183/16000617.0012-2019. PMID: 31694838; PMCID: PMC9488507.
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Amazon Music | Android | iHeartRadio | Podcast Index | RSS | More