This week we are absolutely thrilled to be joined by three Interstitial Lung Disease experts to discuss the workup and differential for a patient with a new presentation of suspected ILD. This is also our first episode in a collaboration between the Pulm PEEPs and the American Thoracic Society Clinical Problems Assembly. In a series of episodes, we will be joined by pulmonary experts from around the country who are leaders in the ATS CP Assembly to provide content on common and cutting-edge topics in PCCM.
Meet Our Guests
Sonye Danoff is an Associate Professor of Medicine at Johns Hopkins and is Co-Director of the John Hopkins Interstitial Lung Disease and Pulmonary Fibrosis program. She also serves as the Assembly Chair of the Clinical Problems Assembly for the American Thoracic Society.
John Kim is an Assistant Professor of Medicine at UVA and has both clinical and research expertise in interstitial lung disease with a focus on pulmonary fibrosis.
Shweta Sood is an Assistant Professor of Medicine at Penn Medicine whose expertise is in Interstitial Lung Disease. She is an integral part of fellowship training where she leads the monthly ILD conference for fellows as well as provides didactics for ILD cases.
Consult Patient
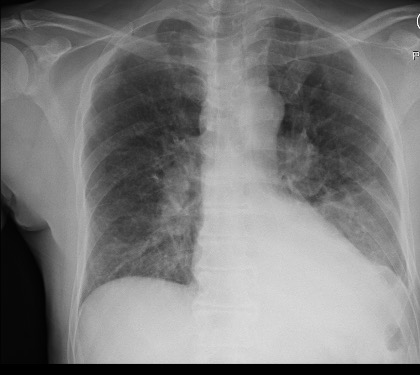
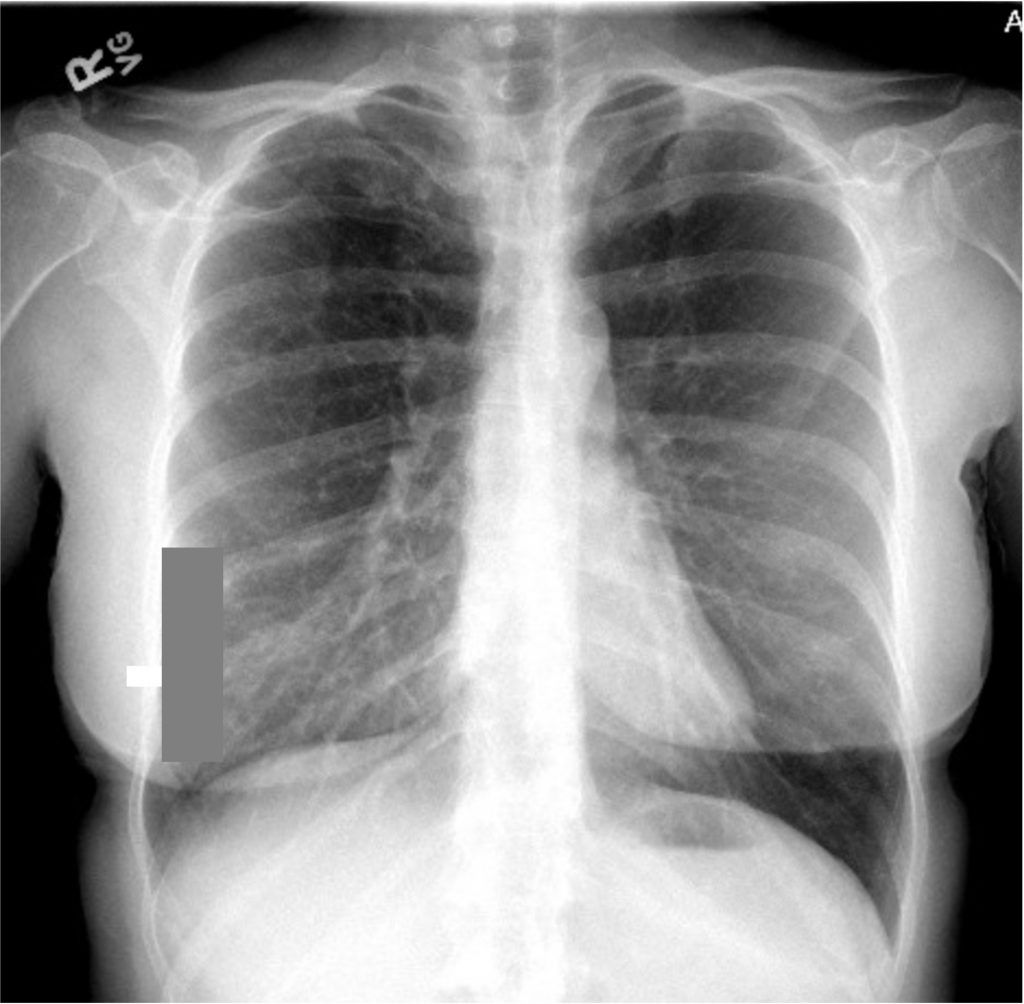
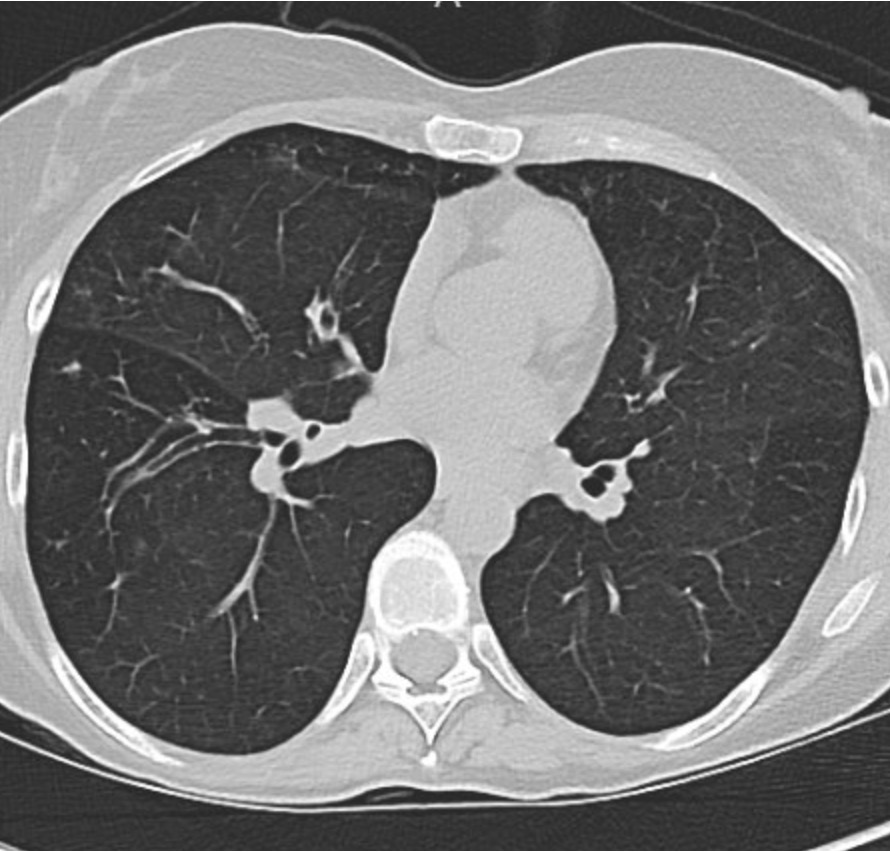
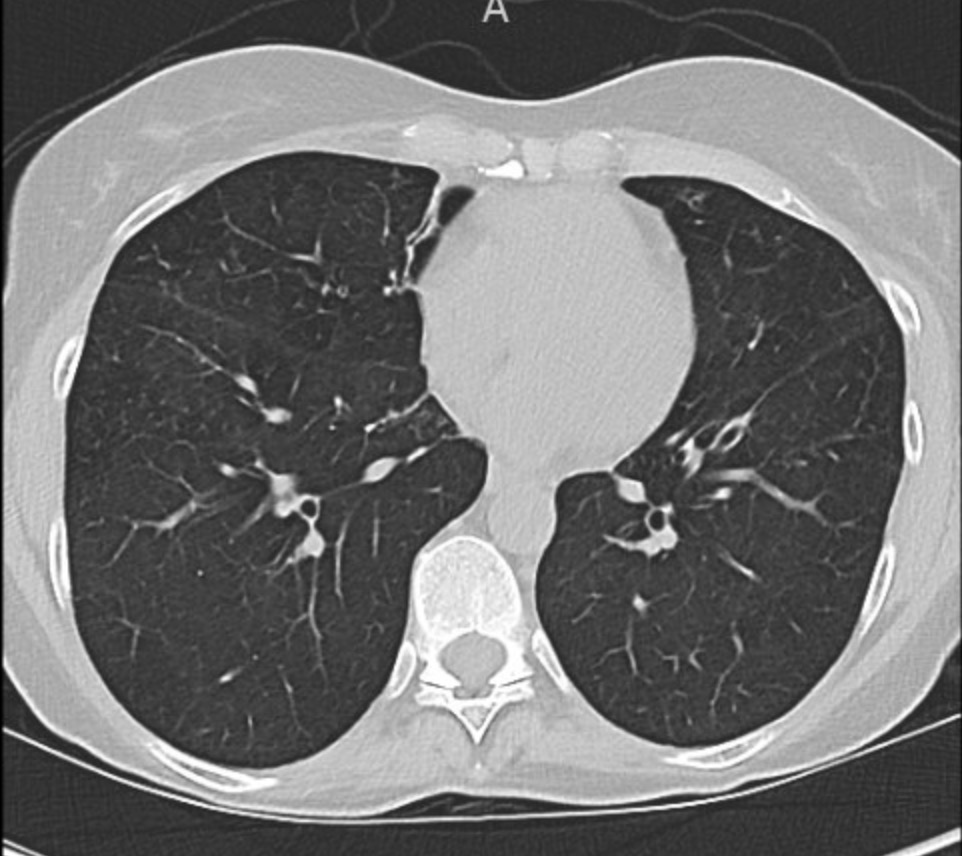
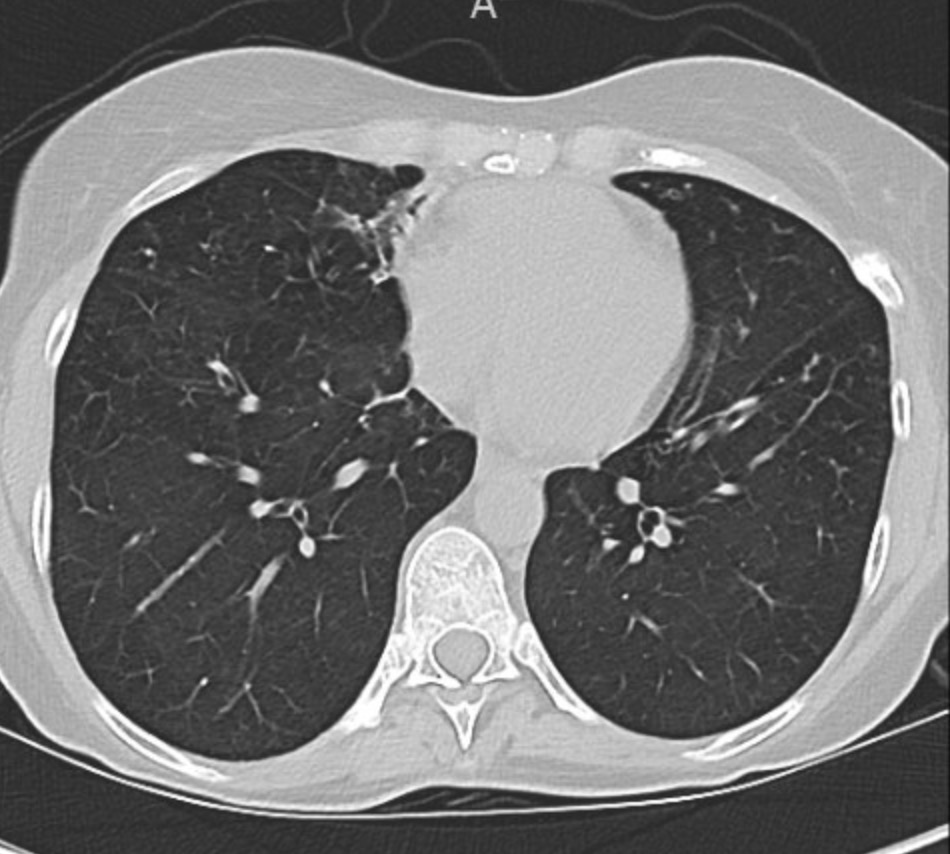
A 66-year-old man who is a never smoker with a past medical history of hypertension and osteoarthritis was admitted to the hospital after presenting with progressive dyspnea on exertion to dyspnea at rest and was found to be hypoxemic. He reports 4 months of progressive dyspnea on exertion but on further questioning, thinks he was last normal about 1.5 years ago when he could walk 2 miles at a time. Currently, he can only walk 0.25 to 0.5 miles before needing to stop. He reports an intermittent, dry cough throughout the day that is not associated with eating, position, or sleeping. A full ROS is negative including for rashes or joint pains. His family history is notable only for hypertension and hyperlipidemia. He is a never smoker, drinks in moderation 1-2 x a week, and lives in the suburbs with his wife. His house has central heating and air conditioning, they have no pets, and they have carpeted floors. He is a retired police detective.
His physical exam is notable for fine crackles at the bilateral bases on pulmonary auscultation, and he is breathing comfortably on nasal cannula although mildly tachypneic to 18 breaths per minute. He has no signs of volume overload, no peripheral clubbing, no rashes, and joint exam does not reveal swelling or synovitis.
Key Learning Points
Take away points from our guests:
— ILD is a symptom, not a diagnosis
— The first time a patient is evaluated for interstitial lung disease is the best chance for making the diagnosis so take the time to evaluate them thoughtfully
— Start the physical exam with the hands first. The hands can reveal a lot about the patient (clubbing, cyanosis, joint, skin, and nailbed findings) and it establishes a personal connection
— When doing the pulmonary exam, percuss first from top to bottom to learn the size of the lungs, and then listen from bottom to top
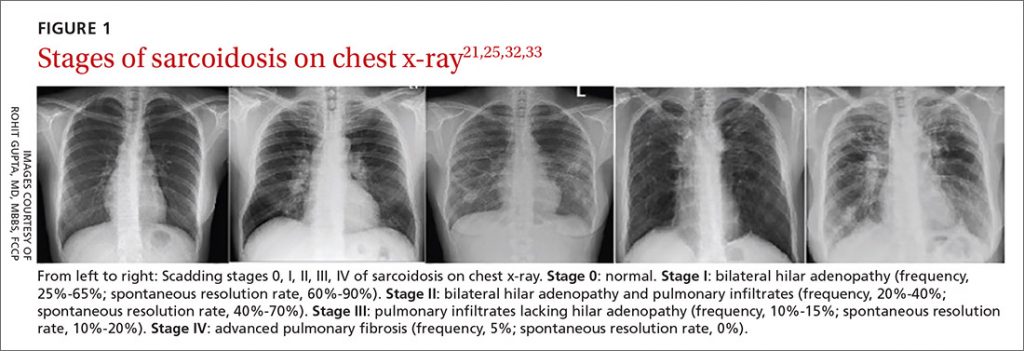
— When reading a CT scan the simplest approach is “Is it a UIP pattern or not?”. This can be your first diagnostic divide. UIP is consistent with IPF, and in select circumstances connective tissue disease, occupational lung disease, or advanced hypersensitivity pneumonitis. Non-UIP patterns have a broader differential
— A multi-disciplinary interstitial lung disease conference is the gold standard for establishing an ILD diagnosis
Gathering the history:
— Ask about onset: acute or chronic. “When was the last time your breathing was entirely normal?”
— Symptoms can be shortness of breath, a lingering cough, or often fatigue and decreased energy. Occurrence is important! Do symptoms occur only with exertion or at rest too?
— “Have you ever had chest imaging before?”
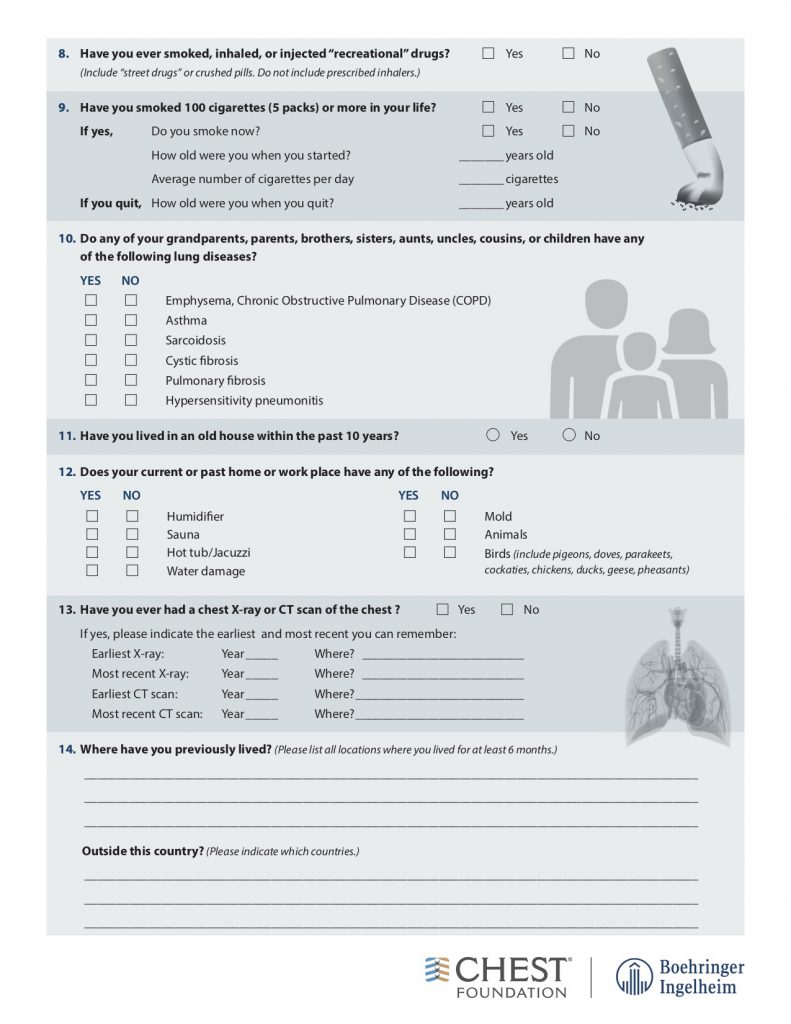
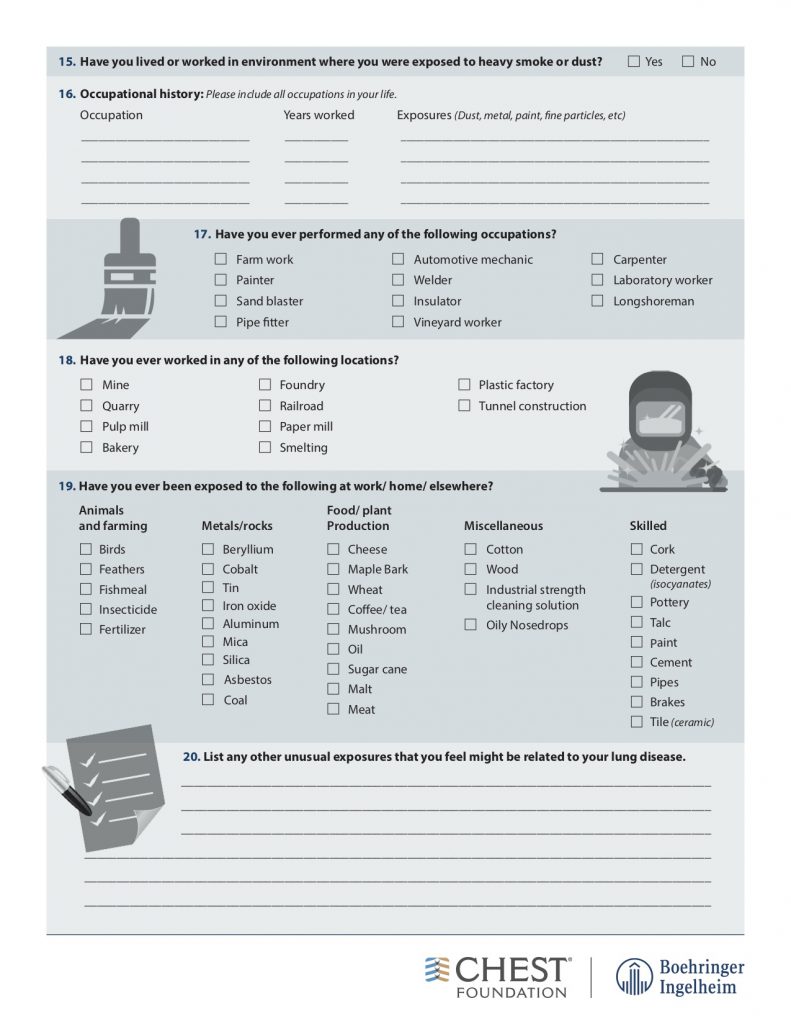
— Take a thorough exposure history, and the weird questions are all necessary! Ask about birds and feathers (pet birds, bird feeders, down pillows or blankets, hunting, taxidermy), mold or water damage, organic or inorganic compounds from work (landscaping, ship yards, coal mines)
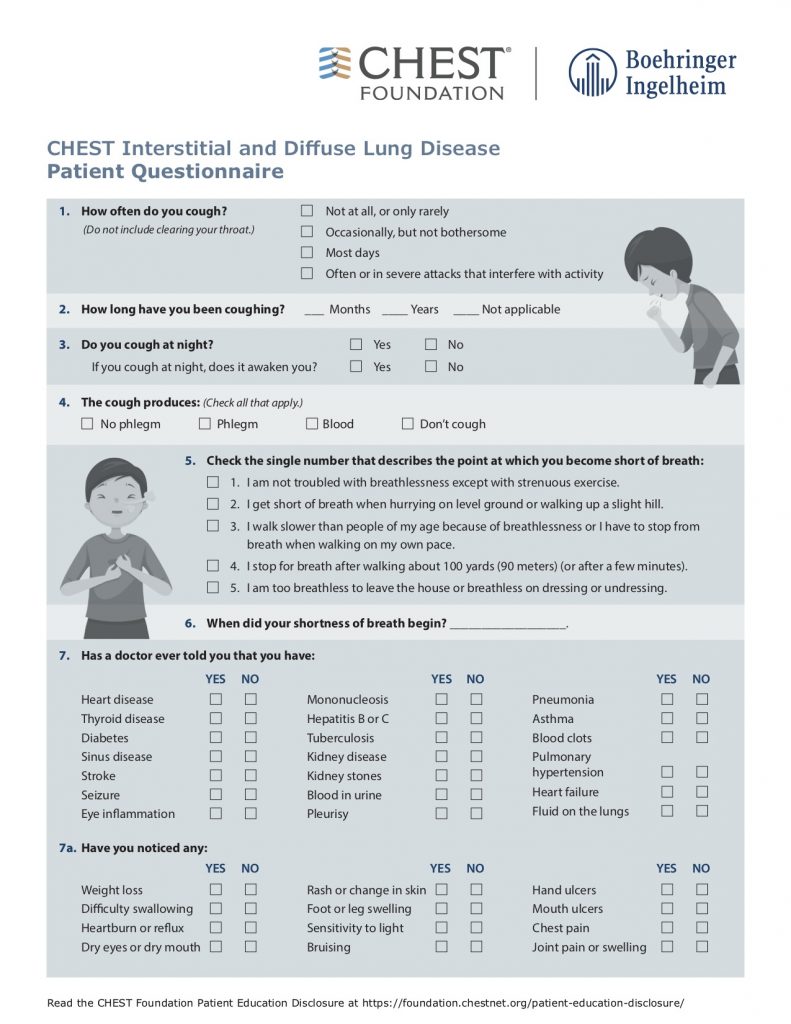
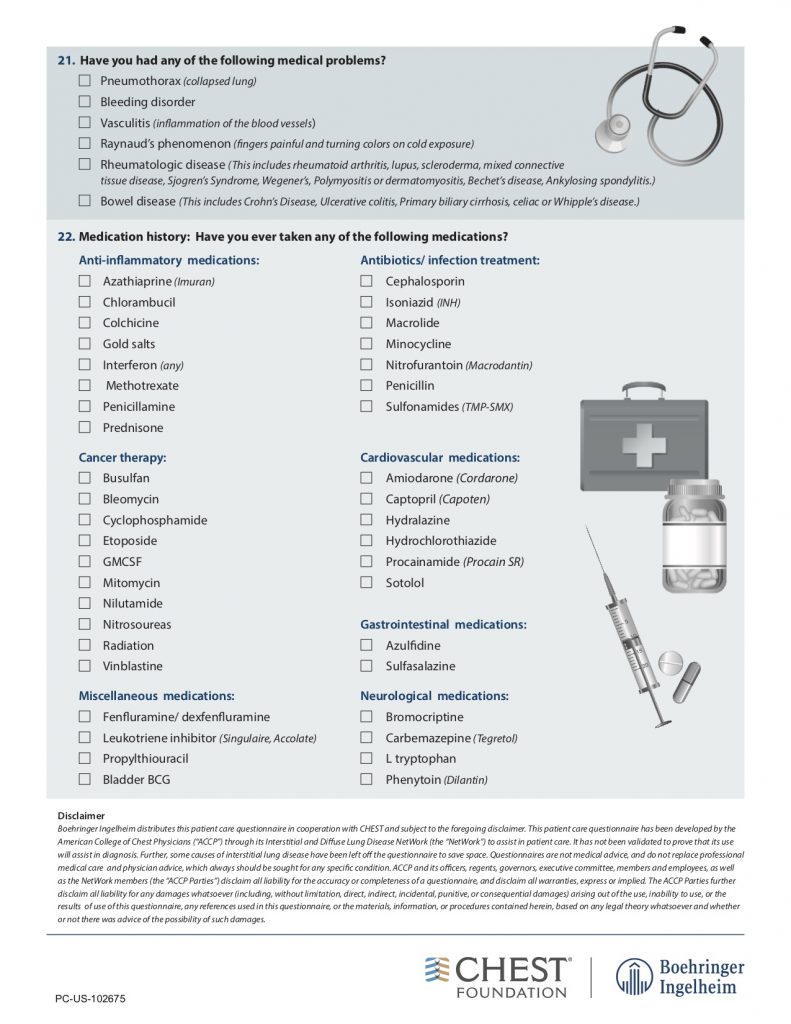
American College of Chest Physicians – Interstitial Lung Disease Patient Questionnaire
Physical Exam:
— Assess stability first and foremost
— Lung findings: crackles, inspiratory squeaks (often in hypersensitivity pneumonitis)
— Look for evidence of alternative diagnoses: volume overload, liver disease, signs of infection
— Evaluate for signs of connective tissue disease: examine the skin around the forehead and mouth for signs of scleroderma, look for rashes, perform a thorough joint examination and look at their hands, and assess muscle strength
Imaging:
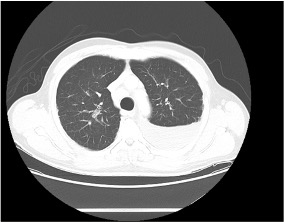
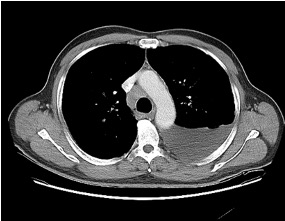
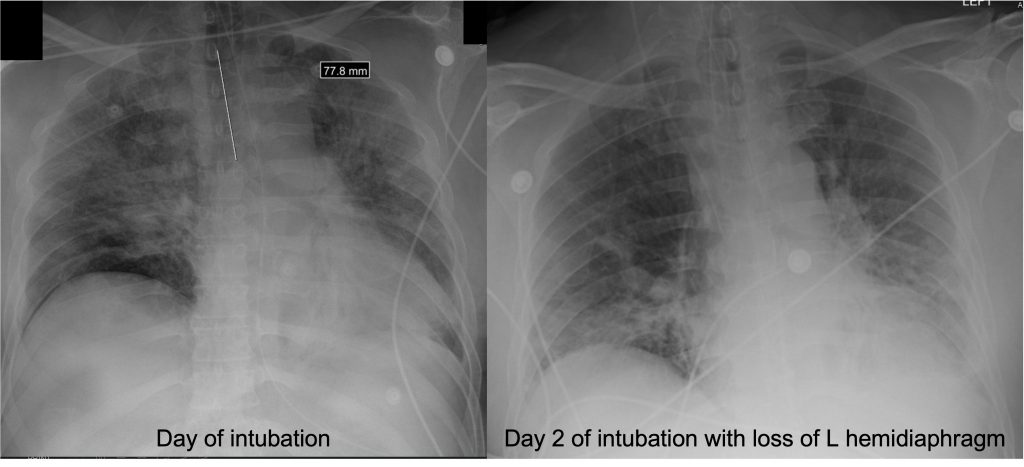
— Order a high-resolution CT scan without contrast. High resolution means thin slices that are 1-2 mm thick. Contrast should be avoided if possible because it makes looking for subtle reticulations or ground-glass opacities harder
— Inspiratory and expiratory films help evaluate for gas trapping. If present, this may indicate hypersensitivity pneumonitis
— Prone films allow you to distinguish reticular changes in the dependent portions of the lungs from atelectasis
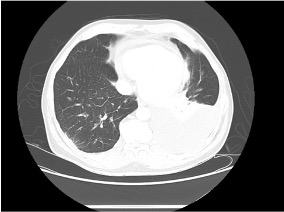
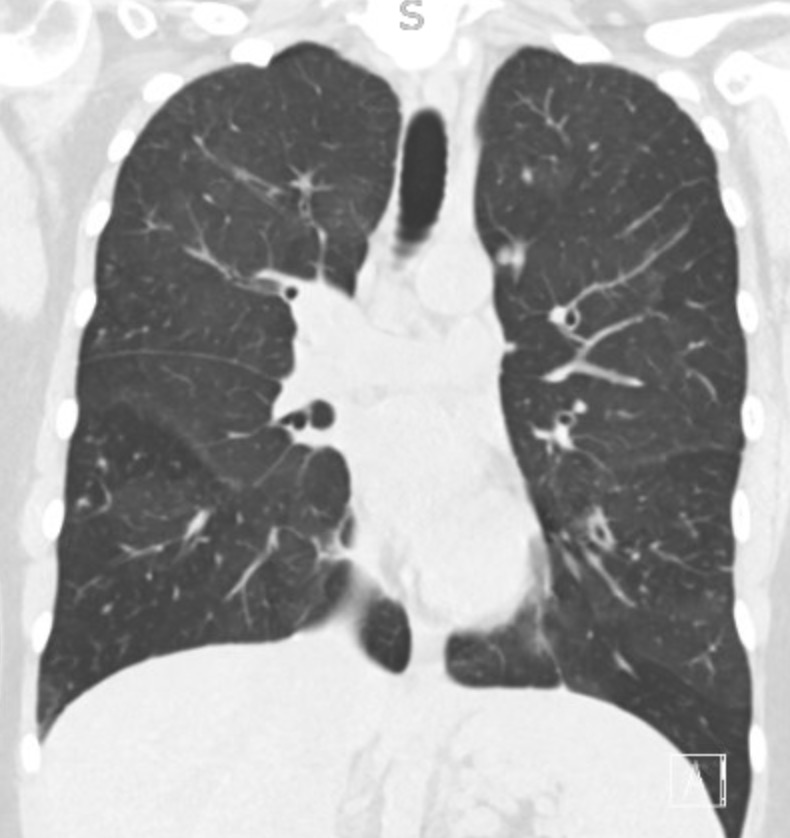
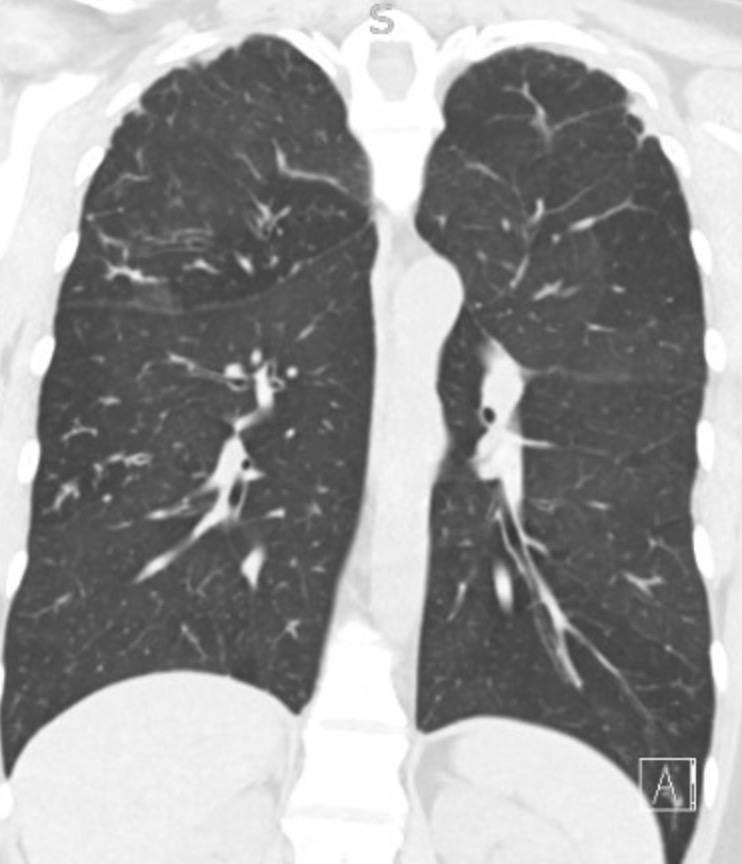
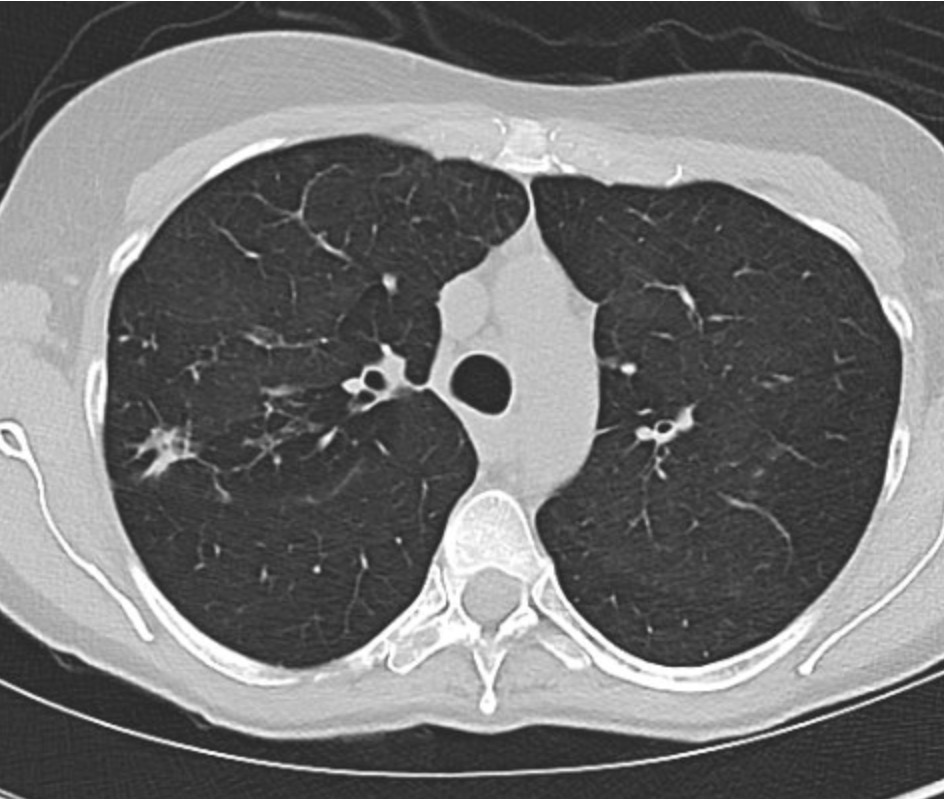
Reading the CT scan:
— Look at the distribution first. Is it uniform from top to bottom or not? Is it subpleural, peripheral predominant, or central?
— Identify key features: reticulation, traction bronchiectasis, honeycombing, ground-glass opacities, cysts, and nodules
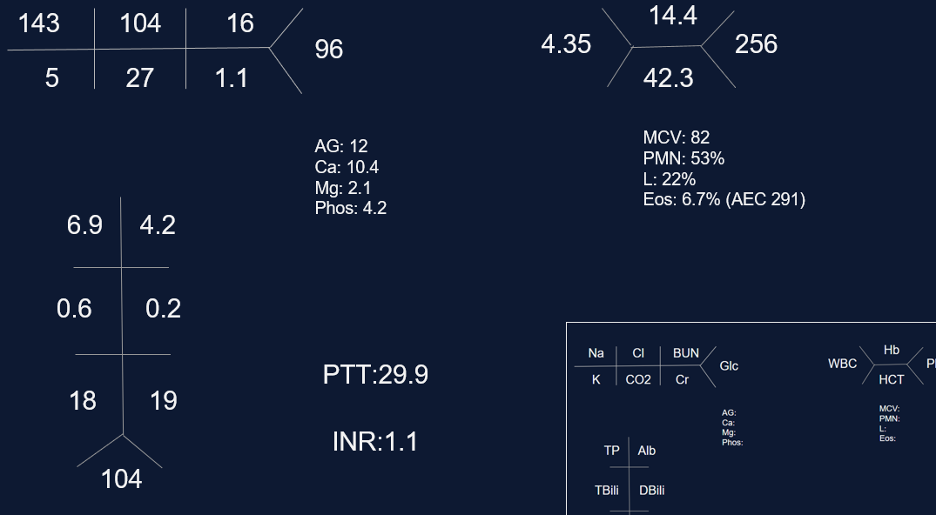
Laboratory evaluation:
— ANA, Scl-70, DS DNA, anti-RNP, anti-centromere, RF, CCP, SSA, SSB, RNA pol 3, HIV, myositis panel, Hypersensitivity pneumonitis panel
Pulmonary function tests:
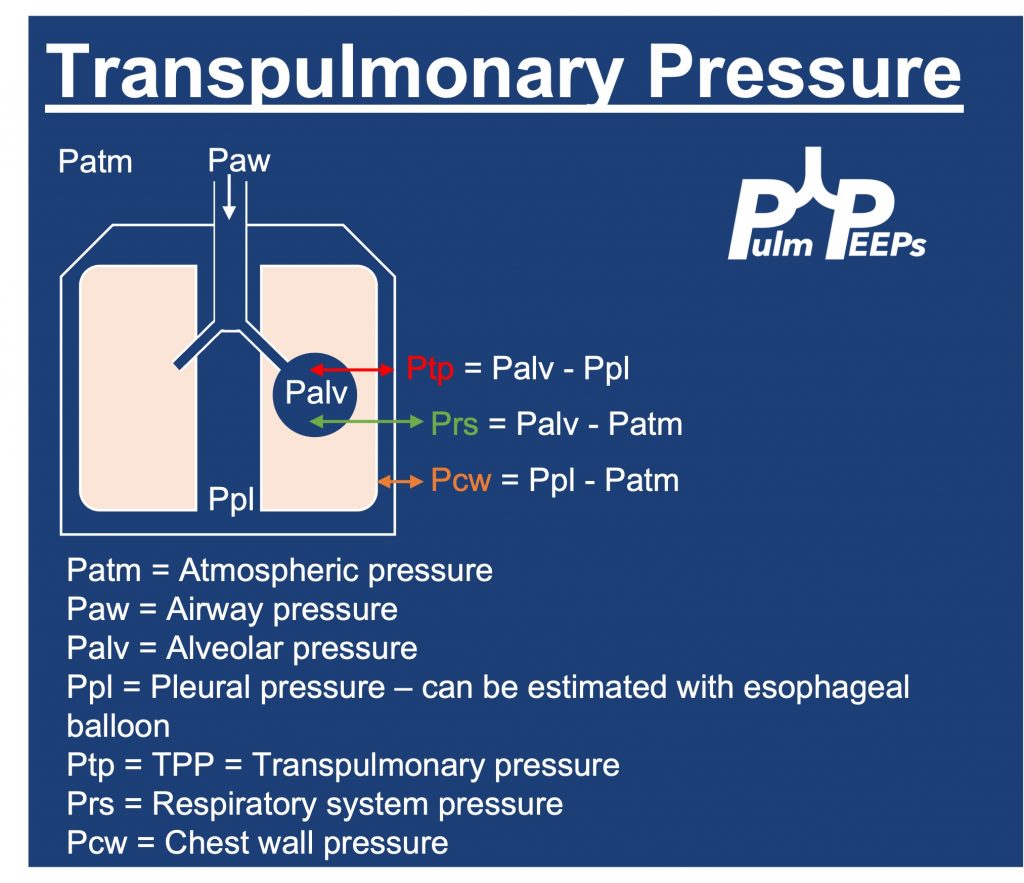
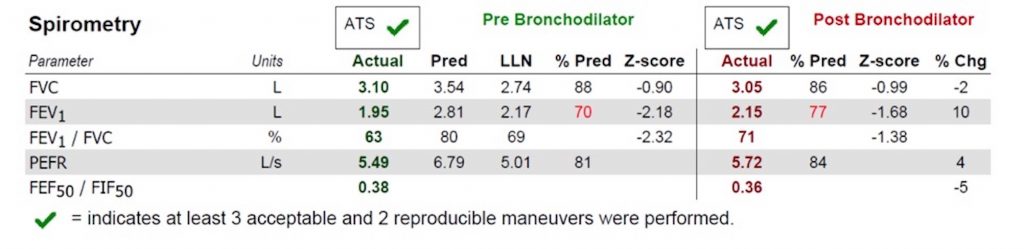
— A restrictive ventialtory defect is the classic pattern and can tell you about severity. In ILD, TLC, VC, FRC, and RV are generally all reduced in proportion
— Identify if there is obstruction or not, because if present this may indicate coexisting emphysema or hypersensitivity pneumonitis
–The DLCO can be helpful for disease severity, and for raising concern about other co-existing diagnoses such as pulmonary vascular disease, or emphysema
— A DLCO < 50% predicted may predict that the patient will need oxygen with exertion and the patient should be walked
6 Minute Walk Test:
— This should be performed for all new patients because it is important for prognosis
— In the first year after diagnosis, a 6MWD should be performed every 3 – 4 months to assess disease trajectory. It can be done every 6 – 12 months after that.
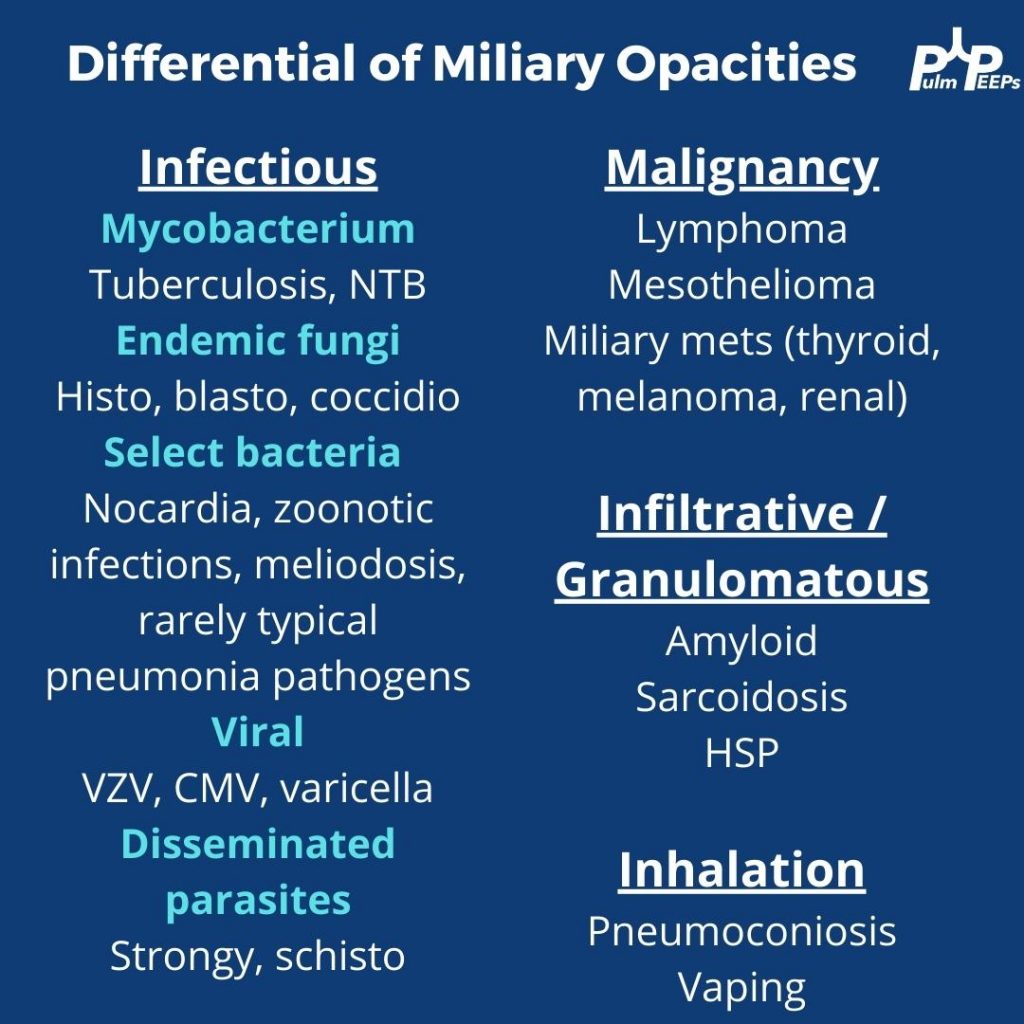
Differential Diagnosis:
Silo ILDs into two large buckets
1) An exposure, trigger, or underlying cause is present: hypersensitivity pneumonitis, medication-induced, occupational lung disease, connective tissue disease, granulomatous disorder
2) Idiopathic interstitial pneumonia
References and links for further reading
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. doi:10.1164/rccm.2009-040GL
- Raghu G, Brown KK. Interstitial lung disease: clinical evaluation and keys to an accurate diagnosis. Clinics in Chest Medicine. 2004;25(3):409-419. doi:10.1016/j.ccm.2004.05.007
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63 Suppl 5:v1-58. doi:10.1136/thx.2008.101691
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277-304. doi:10.1164/ajrccm.165.2.ats01
- Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. New England Journal of Medicine. 2018;378(19):1811-1823. doi:10.1056/NEJMra1705751
- Travis WD, Hunninghake G, King TE, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008;177(12):1338-1347. doi:10.1164/rccm.200611-1685OC
- Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. New England Journal of Medicine. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Hariri LP, Roden AC, Chung JH, et al. The Role of Surgical Lung Biopsy in the Diagnosis of Fibrotic Interstitial Lung Disease: Perspective from the Pulmonary Fibrosis Foundation. Annals ATS. 2021;18(10):1601-1609. doi:10.1513/AnnalsATS.202009-1179FR
- Exposures. hpLung. Accessed February 12, 2022. https://www.hplung.com/
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Amazon Music | Android | iHeartRadio | Podcast Index | | More